16. Chemical Self-Organization
A chemistry teacher is very familiar with the self-organization of matter into more complex forms. That is what chemistry is all about. What the chemist does is to arrange the environment, set the initial conditions, so that the molecules will proceed to develop toward the desired product. Molecules do this self-assembly as they respond to the conditions. The organizing principles are the laws of electrostatics with their production of chemical bonding. What is to be pointed out here is the more basic self-organization of the elements themselves. The natural outcome of the self-organization of the atoms is the emergence of the order of the Periodic Table of the Elements.
Definitions
Element – a pure substance of which all the parts (atoms) have the same structure and properties and cannot be separated into parts without losing their structure and properties. Occurring in nature in 92 different forms – from hydrogen to uranium.
Periodic law of the elements – the chemical and physical properties of the elements are the periodic functions of their atomic number and electronic configurations. The chemical activities of elements are a result of the number of electrons in the outermost (highest energy or valence) level.
Pauli exclusion principle – no two electrons can have the same set of quantum characteristics in an atom at the same time. Therefore, only 2 electrons of opposite spin can exist in the lowest energy level of an atom. Other electrons fill successively higher energy levels in orbitals governed by this exclusion principle.
Orbitals – the sub-levels of the energy levels that surround the nucleus of an atom
Octet rule – a general rule for low atomic number elements which states the energy levels of complex atoms above the first tend toward completion with 8 electrons in the outer energy level.
Note on electron spin: Elementary particles have a property akin to angular momentum and physicists describe it by giving it a quantum number although nothing is spinning. Each orbital of an atom can hold only two electrons and they must be of opposite spins.
Background
The organization of atoms from the elementary particles is an example of self-organization on the most basic physical level. A number of rules and principles have been developed to explain the self-organized complexity of chemical combination.
A necessary condition for the existence of matter is that it form stable configurations. Stability, of course, is a relative term since the time intervals involved are not rigidly defined. We do not expect a volume of helium atoms, for example, to disintegrate as we attempt to determine its mass. Trying to determine the mass of a quantity of uranium 227 is a little more difficult since half of it decays into other elements in minutes.
The number of relatively stable arrangements of elementary particles that self-organize themselves into atoms is limited to the ninety two elements and their isotopes. But when atoms combine into molecules the number of possible arrangements is virtually unlimited. The discovery that just three particles (proton, neutron and electron) can generate all the ninety two varieties of matter and the infinite number of molecular combinations that can be made from them is one of the supreme intellectual achievements of humankind. The Periodic Table of the Elements should be recognized as one of the great masterpieces of scientific accomplishment.
Discussion
It is by observing the behavior of atoms of an element in the presence of other substances that the chemical properties of the element can be deduced. We know now that the chemical behavior of the elements is determined by the arrangement of electrons in the atom’s outermost energy level or shell. The Pauli exclusion principle accurately describes the way atomic structure is organized. The innermost energy level of an atom is filled when there are two electrons in it. The second level requires eight electrons to fill it. When an atom has more than eight electrons in the third level a fourth level begins to form for the ninth and tenth electron and the third level begins to hold more electrons. As electron clouds assemble around nuclei containing many protons, the third level can be forced to hold up to eighteen electrons.
The order of filling of the fourth and higher energy levels gets even more complex. By spectroscopic examination it has been discovered that energy levels have sub levels and that within each sub level there are multiple paths called orbitals. Each orbital can only hold two electrons. And true to the Pauli exclusion principle they are of opposite spin.
Self-organization of atoms – Stellar nucleosynthesis
Matter began to self-organize itself within a fraction of a second after the big bang that initiated our universe. It is then that quarks coalesced into protons and neutrons and when a proton could capture an electron hydrogen formed. Helium nuclei formed when the strong energy input from the initial big bang or the tremendous temperature and pressure in the interior of stars overcame the mutual electrostatic repulsion of protons. The energy forced the protons so close together that the strong nuclear force could act and bind them into atomic nuclei.
The self-organization of atomic nuclei continues within stars as hydrogen nuclei are fused into helium nuclei and the hydrogen core shrinks. These stellar interiors heat up as energy is released from nuclear fusion reactions. Reactions that were not possible before begin. Carbon nuclei form in that moment when two helium nuclei are briefly forced together and are struck by a third. An oxygen nucleus forms when carbon is struck by another helium nucleus. The ordering principle that leads this self-organizing process of nuclei production is that by which systems tend to rearrange themselves into more stable configurations, or lower energy states. The process is called stellar nucleosynthesis.
The process continues within the stars. Oxygen nuclei are bombarded by other nuclei and it is transformed into silicon and sulfur. More fusion accompanied by the decay of some unstable combinations produce different nuclei all the way up to iron. There the stellar furnaces, like that of our Sun, reached the limit of their productive capability. The iron nucleus is the most tightly bound of all the elements.
Heavy element production – Supernova explosion
Up to this point massive amounts of nuclear energy are released as lighter nuclei fuse together. To make nuclei heavier than iron requires energy input. Nature does it with another bang. A supernova explosion of intense pressure synthesizes new heavier elements and spreads them out into the universe. Some are radioactive and begin to break down immediately. But through neutron bombardment the nuclei of all 92 of the chemical elements are eventually made. Thus does nature organize the building blocks of everything.
Atomic formation
Once nuclei are formed and escape the heat of their star mothers, the organizing principle of electromagnet attraction takes over the task of atomic organization. Each nucleus picks up a crowd of electrons equal to the number of its protons and an electrically neutral atom is formed. Because electrons have a dual nature and sometimes behave like waves rather than particles they can exist only at distances from the nucleus that fit their whole wave length. Thus electrons surround the nucleus in discrete energy levels or “shells.”
There is a tendency for atoms to complete the electron capacity of their outermost energy level. They do this by losing, borrowing or sharing electrons with other atoms. Therefore, how an atom reacts with other atoms, what it can combine with and what it will reject is determined by the structure it presents to the world of atoms, that is, by the number of electrons in its outermost energy level. Elements with similar electron structure will have behaviors in common and can be put into family groups. It is the structure of the atoms which determines their chemical properties and their behavior and their grouping in the Periodic Table.
The atomic nucleus can be thought of as an evolving entity as it is transmuted within the heart of stars into increasingly complex structures. This is a form of emergent evolution. Here the term evolution is used in the broad sense as the process through which new structures, behaviors, or concepts come into being from previous ones.
Self-organization of compounds by chemical bonding
Compounds form when atoms combine. As mentioned above, atoms tend to interact in such a way as to wind up with completely filled outer energy levels. They either lose or gain electrons from their reaction partners (ionic bonding).
A common example of ionic bonding is the combination of sodium and chlorine. The sodium atom has a single electron in its outer energy level. It is a very reactive metal. The chlorine atom has seven electrons in its outer energy level. It lacks one electron to make the level complete. It is a poisonous gas. When the two reactants come in contact, chlorine atoms grab the outer electrons held only loosely by the sodium atoms. This makes each sodium atom an ion with an electric charge of +1 and each chlorine atom an ion with a charge of -1. The opposite charges bind the ions together into a crystal of common salt with all its well-known beneficial properties. In a similar way all the multitude of chemical compounds form, each possessing its own set of properties.
In other cases of chemical bonding electrons in the outer energy levels are shared so that both atoms have filled energy levels (covalent bonding). The water molecule is the result of this covalent bonding. The oxygen atom has six electrons in its outermost energy level. Two more will complete it. Hydrogen atoms have one electron surrounding their nuclei. This inner energy level of hydrogen would be complete if one more were within it. Two hydrogen atoms and one oxygen atom can strike a deal. If electrons from the two hydrogen atoms visit the oxygen’s outer energy level and two of the oxygen’s electrons can spend time around the hydrogens’ nuclei all three atoms can have filled energy levels.
By both ionic and covalent bonding atoms get attached to each other and form more complex structures with new properties.
The Periodic Table of the Elements
Of all the intellectual constructions of human culture the periodic table is surely one of the greatest. It stands with Darwin's theory of evolution, as a tool that brings understanding to a chaotic mass of knowledge. It explains how the elements are related to each other. It displays the pattern of order out of which the world is built. Today we account for its regularity with our knowledge of atomic structure, but when it was first proposed by Dmitri Mendeleev in 1869, atomic structure was unknown. He worked by associating properties of the elements with their increasing atomic masses. There was no apparent reason why the properties of various elements should repeat themselves as they did. There had to be a deep connection.
Patterns often reveal laws of organization and the periodicities in the table challenged scientists to explain them. Their search for a physical reason for those periodicities produced the modern atomic theory.
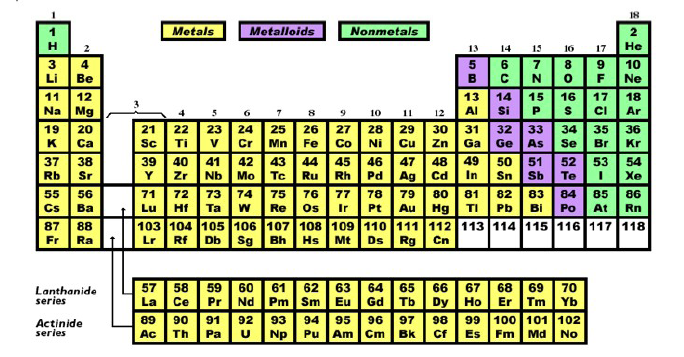
The periodic table is now an everyday working tool of chemists and a common feature on the wall of chemistry classrooms. The fact that it codifies so much information in such a concise presentation obscures its remarkable significance. In it is described the structural organization of atomic nature. It allows us to see, step by step, system by system, how matter becomes increasingly complex. By using the knowledge embedded in the table, chemists have been able to construct new compounds with new properties. The table is a concise statement by the human mind of the order found in nature.
Self-organization of carbon compounds
It is the unique organization of carbon atoms that makes living organisms possible. The six protons in the nucleus dictate that there shall be six electrons in the energy levels surrounding the nucleus. The Pauli exclusion principle decrees that there will be two electrons in the inner energy level leaving four in the outer energy level. This is just half of what is necessary to complete the energy level. Each carbon atom can therefore form four covalent bonds, more than any other element.
The ability of carbon to form long chain molecules by bonding to hydrogen and itself forms the economically important hydrocarbon class of compounds.
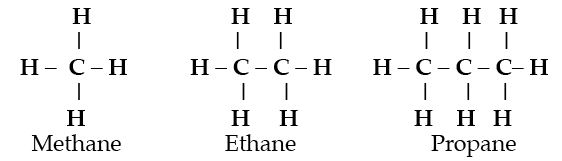
When carbon, hydrogen and oxygen combine, many new compounds like alcohols, sugars, and fatty acids originate. Throw nitrogen into the combination and amino acids and the proteins of living organisms form. Each of these compounds has distinctive properties which cannot be deduced from the properties of the elements from which they are made. They are emergent properties.
It is an interesting discovery that complex hydrocarbons, as well as water, carbon monoxide, ammonia and small amino acids, are found to have formed in interplanetary space. The origin of living organisms on Earth may have been aided if some of the molecules important to living organisms rained down on our young planet from space.
Self-organization of complex protein molecules
Within the living cell proteins are assembled as long chains of amino acids. The sequence of amino acids in the chain is determined by the sequence of the DNA in the cell. This is called the primary structure of the protein. Electromagnetic forces along the chain can change the single strand of amino acids into a helix shape or into a sheet with pleats producing the secondary structure of proteins. They are important to the structure of living organisms because they form the fibrous proteins. One third of all protein in vertebrates is made up of collagen, a fibrous protein. It is a major part of tendons, ligaments, bone, cartilage and skin.
Other electromagnetic forces can twist the amino acid chain into an intricately folded, three dimensional shapes; the tertiary structure. These proteins are the globular proteins with complex biological functions. They are the enzymes, antibodies, and membrane receptors without which living organisms could not exist.
The folding process that self-organizes complex protein molecules does not stop with the formation of tertiary structures from one kind of long amino acid chain. Many proteins start out from combinations of more than one chain and then are subject to the folding process. As many as four different chains may be the source of a molecule.
The insulin hormone is an example of a protein formed from two different amino acid chains. The hemoglobin molecule is a product of four chains. When two or more chains organize themselves into molecules the quaternary structure of proteins is formed.
Summary – the importance of chemical complexity
It is obvious that this change in structure of complex molecules is self organized, necessary for life, produces new structures and new properties.
The premier self-replicator molecule is the now famous DNA molecule. The explanation of how the double helix splits open and the replication of both strands of DNA takes place is the triumph of molecular biology. The understanding of the functioning of DNA and how it codes for the production of inherited properties is the key to the understanding of the evolution of all living things